Overcoming Manufacturing Complexities with Dip Molding
Complex curves and intricate geometries in medical device design can often lead to manufacturing headaches.
When our client approached us with exactly this challenge – a neonatal CPAP cannula requiring precise curvatures – we knew traditional injection molding wasn’t the answer. Instead, we turned to dip molding – a versatile manufacturing process that excels at creating complex devices with challenging contours.
Read on to see how we transformed a complicated design problem into a production-ready success story!
How Does Dip Molding Work?
Dip molding is a manufacturing technique that creates plastic parts by dipping heated metal molds (mandrels) into a liquid polymer like liquid PVC, or latex. It’s commonly used for medical balloons, cannulas, and surgical gloves. (All complexly shaped items with curves!)
Let’s walk through the process step by step:
1. Designing and Manufacturing the Mandrels
- The first step involves designing and modeling the metal molds, known as mandrels.
- Mandrel design is crucial. A large radii is needed to facilitate the de-molding process, and there should be enough extra space left at the top of the mandrel to allow for the manufacturer to grip the mandrel while dipping.
- After the design is complete, the mandrels are manufactured on a CNC machine from either aluminum or steel.
- For finishing, electropolishing is recommended since it facilitates the de-molding process by creating a smoother internal part cavity.
- When the mandrels are finished, they are sent to the dip molding facility.
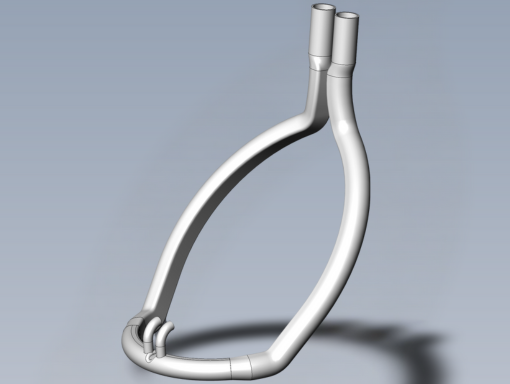
This is a CAD visual of a mandrel designed to produce cannulas.

2. Dipping and Curing
- At the dip molding plant, heated mandrels are dipped into the liquid polymer.
- The most commonly used polymer is liquid PVC or plastisol, with other options available like latex, polyurethanes, and silicones.
- Dipping can be done manually by hand or with a programmable, automated machine. Automation enables more precise control over things like orientation of the mandrel and speed of dipping. This results in more consistent, repeatable results.
- The final thickness of the product is determined by several factors like the initial mold temperature, polymer temperature, dipping speed, and dwell time (how long the mandrel stays submerged).
- After a set dwell time, the mandrels are placed in an oven to dry. The heat from the oven cross-links the polymer, curing it into a solid piece.
3. Demolding and Finishing
- Finally, the mandrels are left to cool, and then the finished parts are removed from the mandrels.
- The parts are now ready to use!
Finding this info helpful? Sign up for more!
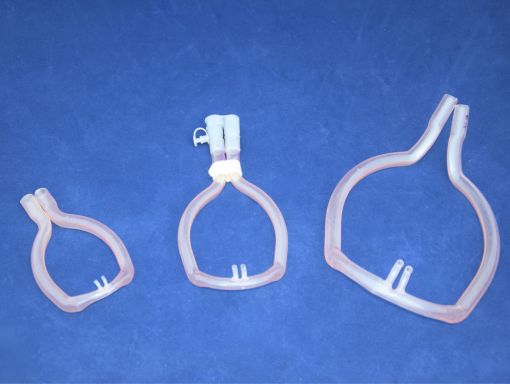
What are the Pros and Cons to Dip Molding?
Benefits
1. Cost Savings
- Lower tooling and production costs compared to injection molding.
2. Time-to-Market Advantage
- Quick turnaround time allows for rapid protoyping
- Freedom of design
3. Scalability
- Works for small or large production runs
- A wide range of materials with varying durometers can be used.
- Finished parts have a seamless appearance suited for distribution.
Limitations
1. Potential Imperfections
- Drip marks or bubbles are possible on some samples due to the nature of the process.
2. Design Constraints
- It’s necessary to maintain consistency in the wall thickness of parts, which can be tricky.
- The mandrels need to be designed in specific ways if one consistent piece is desired.
Transforming Medical Device Manufacturing Through Dip Molding
At Root3 Labs, we’ve helped numerous medical device manufacturers overcome complex design challenges through our expertise in manufacturing methods. Whether you’re developing a new device or looking to optimize or scale your current manufacturing process, our team can help you evaluate if dip molding is the right solution for your needs.
Ready to explore how dip molding could transform your medical device manufacturing? Contact our engineering team today for a free consultation and discover how our dip molding techniques can enhance your medical device manufacturing process.




